Huge energy is stored in the nucleus, and the change of the nucleus (from one kind of nucleus to another kind of nucleus) is often accompanied by the release of energy.
At noon, when we are outdoors, we will feel the blazing sun, hot and dazzling, and even beat raw eggs on the metal after the sun shines, and the eggs will be cooked after a while. This energy of light and heat comes from the nuclear fusion that occurred inside the sun hanging overhead, but this energy was sent from the core of the sun 10 million years ago, and it took 8 minutes to reach the earth.
The constantly fusing sun
The temperature at the center of the sun can reach 15 million degrees Celsius, and the pressure is equivalent to 300 billion atmospheres. Under the huge pressure, the sun uses 600 million tons of hydrogen atoms per second to participate in fusion, of which 4 million tons of matter are converted into energy. A thermonuclear reaction in which four hydrogen nuclei fuse into one helium nucleus is going on.
In a nuclear fusion chain reaction, two hydrogen nuclei (protons) fuse to emit a neutron and a neutrino, creating a helium-2 nucleus. The helium 2 nucleus reacts with a hydrogen nucleus to emit a gamma photon to generate a helium 3 nucleus. Two helium 3 nuclei fuse to form a stable helium 4 nucleus and release two protons at the same time. During the whole process, four hydrogen nuclei fuse to form a helium-4 nucleus, and release two neutrons, two neutrinos and two gamma photons at the same time. Most of the energy released by the sun is carried by gamma photons.
At high energies deep in the sun’s core, helium-3 can merge with an already existing helium-4 to form beryllium-7. Originally, beryllium-7 would find a proton to form boron-8; however, because it is unstable, it has not had time to react and decays to lithium-7 first. In our sun, the decay usually occurs first, followed by the addition of a proton, producing beryllium-8, which immediately decays into two helium-4 nuclei. The helium-4 produced by this process accounts for about 4 14% of the total.
But in more massive stars (for example: O, B class stars), the fusion of protons with beryllium-7 occurs before decaying into lithium, forming boron-8, which first decays into beryllium-8 and then decays into Two helium-4 nuclei. This process is unimportant in Sun-like stars—only 0.1 percent of the total helium-4, but in giant O and B-type stars it is the most important fusion reaction to produce helium-4.
Artificial controllable nuclear fusion device
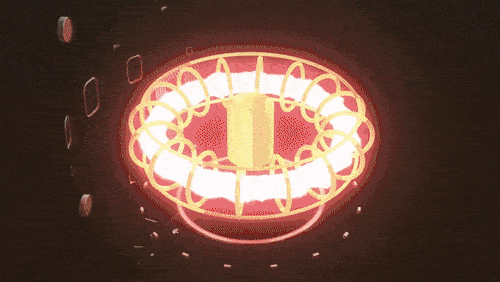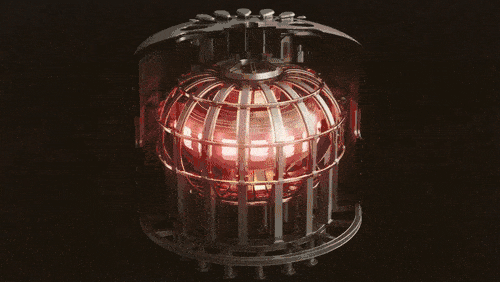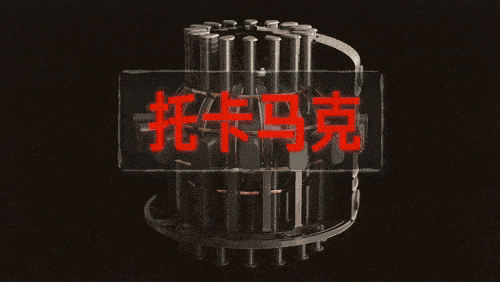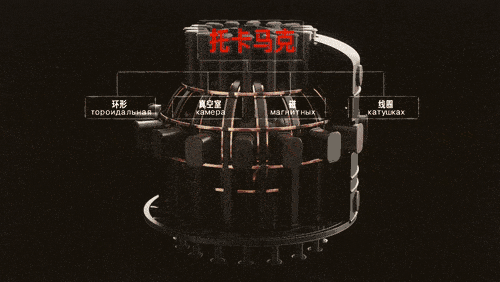
At present, the means by which humans initiate nuclear fusion mainly depends on raising the temperature. Among all nuclear fusions, the nuclear fusion reaction of hydrogen requires the lowest temperature, and the fusion of deuterium-tritium is the easiest to realize.
Elementary primary “processing plant”
After reading the above patiently, did you find anything?
“Hydrogen-helium-lithium-beryllium-boron”
Yes, it is the beginning order of the periodic table of elements in our middle school chemistry!
When the internal temperature of the star rises to 100 million degrees, the helium element will fuse to produce heavier elements, and the fusion process is more complicated. The products are mainly carbon and oxygen, and some neon elements and so on.
When the internal temperature of the star reaches 800 million degrees, carbon begins to fuse; when it reaches 1.5 billion degrees, neon starts to fuse; when it reaches 1.8 billion degrees, oxygen begins to fuse; its products are mainly silicon, and other elements include calcium, sulfur, etc. and other elements.
solar photosphere

For massive stars, the temperature will become extremely high at the end of evolution, which will eventually trigger the fusion reaction of silicon, and the product is mainly iron. Once iron is formed inside the star, it means that the end of the star is coming. At this time The star becomes extremely unstable.
life of a star
For example, the Proxima Centauri next door to our solar system is actually a very low-mass red dwarf. This kind of star can only fuse hydrogen into helium, and yellow dwarfs like the sun are not much better. In the lifetime of the sun, it can only fuse oxygen element with atomic number 8 at most.
The dividing point between fusion and fission – iron element
Every time new matter is created, it is accompanied by a change in the shape of the star. Until nuclear fusion produces iron, nuclear fusion will stop. This is because the specific binding energy of iron-56 is the highest among atoms, which also makes iron lose the possibility of nuclear fusion.
Only those stars with enough mass (10 times the mass of the sun) have the ability to start round after round of nuclear fusion reactions in their cores, and then create heavier and heavier elements, but even such stars are impossible All the known elements in the universe are fused, because the nuclear fusion of stars will stop when the iron element with atomic number 26 is reached.
iron element

This is because the iron nucleus is particularly stable. It means: If you want to break apart an iron nucleus, you need energy, we call this the specific binding energy, and in the entire periodic table, it is the most difficult to break apart an iron nucleus, because the iron nucleus is relatively The nucleus with the highest binding energy.
Scientists have discovered that in nature, the atomic nuclei of elements with atomic numbers before iron can fuse into atomic nuclei of elements with higher atomic numbers and release a large amount of energy. This is the nuclear fusion reaction, and hydrogen bombs are based on this principle. The nucleus of an element with a higher atomic number than iron has a tendency to fission, and it is easier to reduce its own atomic number and release energy through nuclear fission. This is the principle of the atomic bomb.
Iron element is the specific binding energy cut-off point

Only the iron nucleus, if you want the iron nucleus to undergo nuclear fusion reaction again, the conditions are very harsh, and the energy required for the final reaction is much more than the energy produced after nuclear fusion. Nuclear fusion is releasing energy. Only when it comes to the iron nucleus, if fusion occurs, it needs to absorb a lot of energy, which becomes a process that cannot make ends meet. This makes the iron nucleus the most stable element nucleus.
Not only that, those stars that can eventually go to the nuclear fusion reaction of the iron nucleus are generally stars with a mass greater than 10 times the mass of the sun. The stars at this time are called: giant onion stars.

In fact, this is the case. Due to the increase in the atomic number, the conditions for the nuclear fusion reaction of elements will become difficult. The nuclear fusion reaction of hydrogen nuclei only needs 10 million degrees, and the nuclear fusion reaction of helium nuclei needs 200 million degrees, and the formation of iron What about nuclear fusion? You need 3 billion degrees! This is comparable to the temperature at the time of the Big Bang.
Heavy element production line “neutron capture”
Iron element is the end point of nuclear fusion, so how are the elements after iron produced? Anyway, it is impossible to pass nuclear fusion. Another implementation path is called “neutron capture”.
As the name implies, “neutron capture” means that the atomic nucleus captures neutrons. For the convenience of understanding, we can imagine the atomic nucleus as a “snake” game. In an environment with neutron radiation, these snakes may ” Some of the neutrons that come to your door can be eaten, but their “digestion ability” is different, some can even “eat” several neutrons, and some will “eat” only one neutron. indigestion”.
“Snake” game

For example, let’s say an iron-56 nucleus “eats” a neutron, it becomes iron-57, since iron-57 is a stable isotope, so it is fine, in the following time, if it One more neutron is “eaten” and it becomes iron-58, which is still a stable isotope, so it still doesn’t matter.
If it “eats” one more neutron, it becomes unstable iron-59, so it “indigests”, in this case, the nucleus of iron-59 undergoes beta decay, In this process, a neutron in its nucleus decays into a proton, which releases an electron and an antineutrino, increasing its atomic number by 1 and becoming a cobalt-59 nucleus.
The “neutron capture” mentioned above usually occurs in the interior of the star. Since the neutron radiation inside the star is relatively weak, the efficiency of producing heavy elements is relatively low, so this is also called “slow neutron capture”. captured”.
There are “slow neutron captures” in the universe, and of course “fast neutron captures”. In fact, among all known elements heavier than iron in the universe, the contribution of “slow neutron captures” is actually not large. And it is “fast neutron capture” that really produces a large number of such elements.
cosmic extreme event
Extreme events in the universe include supernova explosions and neutron star collisions. When high-energy events such as supernova explosions and neutron star collisions occur in the universe, an environment with extremely strong neutron radiation will be formed in a short period of time, and the order of magnitude can be as high as 100 trillion neutrons per cubic centimeter per second.
In an environment with such a high neutron density, “fast neutron capture” occurs, the lighter nuclei “chow down”, and then severe “indigestion” occurs, so they have all kinds of problems. All kinds of decay, when everything subsided, a large number of elements heavier than iron also appeared in the universe. Such as the silver jewelry we wear, gold and so on. All may come from a high-energy cosmic event.
The distribution of gold on the earth is not small

Supernova explosions are the only way humans know to produce heavy elements, but heavy element resources in the universe are very rich, such as copper, zinc, lead, etc. on our earth, so scientists suspect that there are other ways to produce heavy elements in the universe, Or these heavy elements could have been formed during the Big Bang.
Schematic diagram of a supernova explosion

human body composition
The chemical elements in the human body are ranked from high to low: oxygen, carbon, hydrogen, nitrogen, calcium, phosphorus, sulfur, potassium, sodium, chlorine, magnesium, iron, copper, iodine, manganese, etc. According to the content, it is customarily divided into two categories: macro elements and trace elements.
There are 11 main elements. Their names and contents are: oxygen 65.00%, calcium 2.00%, sodium 0.15%, carbon 18.00%, phosphorus 1.00%, chlorine 0.15%, hydrogen 10.00%, sulfur 0.25%, magnesium 0.05%, nitrogen 3.00%, potassium 0.35% %.
In addition, there are more than 40 kinds of elements that make up the human body, the total amount of which is less than 0.05% of the human body mass. Therefore, these elements are called trace elements in the human body. Trace elements needed by the human body There are eight kinds of trace elements needed by the human body, including iodine, zinc, selenium, copper, molybdenum, chromium, cobalt, iron
。



GIPHY App Key not set. Please check settings